Can MDMA (Molly, ecstasy) damage your brain?
No topic related to MDMA has caused as much controversy as the claim that it damages user’s brains. The short answer is that science has proven (at least in my opinion) that moderate MDMA use does not cause any lasting harm. The long answer…is a very long answer indeed, but worth the read.
In the Beginning…
To really appreciate the debate over MDMA neurotoxicity, it helps to understand a little bit about where these claims came from. In the mid 1980s in the United States, MDMA was starting to be sold very openly and widely. It was still legal, and dealers were doing their best to capitalize on that fact. The Drug Enforcement Agency responded the way it always does when a drug becomes popular: It declared that MDMA was a terrible menace and had to be outlawed. There was just one problem: Federal law requires that a drug be inherently unsafe to use under any circumstances in order for the D.E.A to declare it illegal. If MDMA was reasonably safe, they couldn’t place it in Schedule 1 (the list of the most dangerous and restricted drugs, like heroin.) This proved to be quite a challenge to them, since at the time there wasn’t a single known case of MDMA users being killed, injured, or even addicted. They desperately needed a trump card to justify placing it in Schedule 1. Then, some bright fellow remembered that many drugs were neurotoxic (causing damage to the brain) at high doses; it was likely MDMA would be as well if the dose was pushed high enough.
They needed a ‘scientist’ to do the research, and they knew just who to call: George Ricaurte. Ricaurte was a relatively young and inexperienced researcher, but his work had been very predictable: Every time the government gave him money, he came back with an ‘anti-drug’ research article to support their claims. (Indeed, it’s about the only sort of research Ricaurte seems to have ever done.) Ricaurte performed as expected: He got a grant, they got a paper declaring that MDMA was neurotoxic.
And in fact, MDMA is neurotoxic if you give an animal too much under the wrong circumstances. Just as many other drugs are, such as that Adderall you were given for your attention-deficit disorder, as well as a number of other fairly safe FDA-approved drugs. The real question, then, is not whether MDMA could cause permanent damage; at a high enough dose all drugs cause widespread damage and death. The real question is “how much does it take?”
Ricaurte’s Inter-Species Love Affair
To try to answer that question, Ricaurte embraced an old technique called “inter-species scaling.” It’s a mathematical formula that, based on an animal’s size, tries to predict how toxic a drug will be based on toxicity data from other (smaller or larger) animals. It’s a very widely used approach, and in many cases works well. (Doctors use the same sort of formula to estimate how much of an adult dosage of a drug to give to children/infants.)
But…it’s far from perfect. For interspecies scaling to work, the mechanism by which the drug is toxic must be a simple one. Throw more than one factor into the pot, and things tend to break down. MDMA’s mechanism of neurotoxicity is not a simple one (more on that later). The animal toxicity data is also based on injecting animals with the drug (as much as 50.0 mg/kg, when a human would get plastered at about 2.0 mg/kg.) Injecting a drug bypasses the digestive tract and delivers the drug into the bloodstream much more quickly than taking a drug orally would. As a result, an animal that gets an injection is exposed to much higher peak concentrations of the drug. (In fact, Ricaurte’s own research found that injecting MDMA could as much as triple its neurotoxicity over oral dosing (depending on brain region))[1]In spite of the highly questionable suitability of applying the standard inter-species scaling formula to MDMA, Ricaurte did so, and has been a fanatical defender of it ever since. His most recent claims are that 1.28 mg/kg (a low recreational dose) of MDMA in a human would be detectably neurotoxic.[2]Other researchers disagreed with Ricaurte, condemning his techniques and claims as something other than science…but controversy within academic circles hardly mattered to the government. They got what they paid for: A scientist suggesting that human MDMA users were damaging their brains. It was all they needed. They declared that MDMA had to be placed in Schedule 1 (even after the judge reviewing the case had told them that it legally couldn’t be) and did just that. (Read MDMA History for more info. The entire legal record is available at MAPS.)
Mechanisms of neurotoxicity
Given a large enough dose under the wrong circumstances, MDMA can cause the destruction of the axons of serotonin neurons. (See Your Brain and The Neuron for more information on what serotonin neurons and axons are and do.) The neuron itself (cell body) is not destroyed; only the axon is. (MDMA does not appear to kill brain cells, even under this ‘neurotoxic’ scenario.)
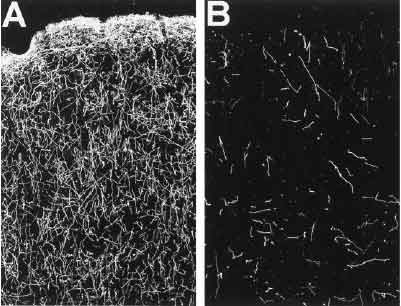
These are before-and-after images of monkey brain frontal lobes. The bright lines are serotonin axons (chemically treated to stand out.) These monkeys were injected with 10 mg/kg of MDMA a day for four days straight by Ricaurte.[16]
How it causes this strangely precise damage has been the subject of a lot of speculation and debate. Given the selectivity of the damage, it seemed likely that something that was damaging to the axons was being concentrated within them. As more research was done, it became clear that the toxic chemical (whatever it was) was being actively pumped in by the serotonin transporters(SERTs.) Many researchers believed (with a good deal of evidence to support the idea) thatdopamine was the toxic chemical, which they thought might be getting in through the SERTs after the local serotonin supply was depleted by MDMA.
In 2002, an important new piece of research was done: Animals who had been given drugs that removed virtually all of the serotonin and dopamine in their brains were given what should have been a neurotoxic dose of MDMA. And nothing happened. There was no damage.[3] At first, it seemed as though the ‘dopamine’ camp had been right. However, the scientists noticed that something else was different in these dopamine depleted animals: When they gave them the overdose of MDMA, they didn’t overheat as expected.
That was significant, because research has shown that body temperature is a critically important factor in MDMA neurotoxicity. It wasn’t enough to give an animal a massive dose of MDMA; if overheating didn’t occur, the animal could usually cope and did not suffer damage. Knowing this, the researchers took another group of dopamine depleted animals, gave them another overdose of MDMA, and warmed them with heating pads so that their temperature reached the same levels as normal (control) animals did when given the same amount of MDMA. The results were striking: The animals with essentially no dopamine in their brains suffered the same level of neurotoxic damage as the control animals did when their body temperatures were kept as high. Something else was at work; dopamine was not the toxic chemical in question.(Ironically, dopamine and norepinephrine release appears to still be important in MDMA neurotoxicity because it promotes activity/heat production, increasing the risk of overheating (which in turn greatly increases the risk of neurotoxicity.))
The “Toxic Metabolite” theory:
In one of the earlier experiments with MDMA neurotoxicity, MDMA was directly injected into a small part of some rat’s brains.[19] The rats didn’t develop noticeable levels of neurotoxicity, which, combined with other clues, led some researchers to suggest that perhaps it was not MDMA itself that was toxic, but rather, that the MDMA was being metabolized (broken down) in the liver, converting into something that was toxic.
In the search for a toxic metabolite of MDMA, the best candidates were various forms of ether-ring cleaved N-demethylated MDMA, such as the glutathione adduct of 3,4-dihydroxyamphetamine (which is indeed neurotoxic.)[20] However, the theory of such ring-cleaved metabolites as the toxic substance falls short in light of reports that animals that were deficient in the enzyme primarily responsible for cleaving the ether ring of MDMA (CYP2D6) did not exhibit any greater resistance to MDMA neurotoxicity (in fact, there were more vulnerable.)[21] Animals given drugs that inhibited this group of enzymes have also been reported to suffer equal levels of neurotoxicity.[22] Glutathione depletion has also not been found to be protective, (although the dihydroxyamphetamine metabolite could be forming conjugates with other amino acids.)[23] Personally, I don’t find the ‘toxic metabolite’ theory a very convincing candidate as a major source of toxicity. Part of the motivation to look for toxic metabolites may have been a red herring; the localized injections into rat’s brains that failed to produce notable damage probably also failed to produce a significant increase in body temperature. Further research is needed.
Gathering the clues:
We still don’t really know what the toxic chemical is. We do, however, have a lot of strange and interesting clues.
• Overheating, often of life-threatening proportions, is important for neurotoxicity to occur.
• Treatments that block an enzyme in the brain called MAO (MonoAmine Oxidase) can completely prevent neurotoxicity.[4] • Whatever the toxic chemical is, it appears to be getting in through the SERT (SSRIs can reduce or prevent MDMA neurotoxicity.)
• MDMA (among other things) is taken up by the SERT. (See MDMA At Work.)
• MDMA is broken down by some enzyme in the brain at a greater rate than in the rest of the body, forming MDA and 3,4-methylenedioxyphenylacetone in significant amounts (both of which represent attacks on the amine, such as MAO might produce.)[24] [25] • Antioxidants like vitamin C and alpha-lipoic acid can reduce neurotoxic damage, even when everything else goes wrong.[5]
The consolidated view:
How could it all be made to fit together? What did these things have in common? There is a common thread:
• The MAO enzymes break down many chemicals similar to MDMA (including dopamine, which is one of the reasons dopamine had been a suspect.)
• When MAO breaks down one of these drugs, it produces reactive oxygen species…chemicals like hydrogen peroxide and superoxide (a more reactive form of oxygen.)
• Your brain has other enzymes that protect it from superoxide and hydrogen peroxide by breaking them down into less reactive chemicals (including water.) As temperatures rise, these enzymes become less effective, and eventually stop working altogether, leaving the reactive oxygen species to run wild and attack the axon.
• Antioxidants are chemicals that, when they run into an oxidizer like hydrogen peroxide or superoxide, will easily react with it, neutralizing it. Antioxidants are part of the body’s natural defense system against such damage.
Under this model, three things need to happen in order for you to suffer neurotoxicity. First, you need to take a fairly large dose of MDMA (how much is needed isn’t clear.) Then, you need to run a very high body temperature for an extended period of time. And finally, the axon’s supply of antioxidants must be largely exhausted.
My personal theory is that the chemical being broken down by MAO is in fact MDMA itself. After all, MDMA is an amine, MAO attacks amines, and MDMA is clearly being attacked at the amine by something in brain tissue. It’s a long-standing tradition in pharmacology that amphetamines are essentially immune to MAO. Resistant, yes. Immune? I don’t buy it. There is simply no reason MAO couldn’t break down amphetamines. (Technobabble: Neither steric hindrance nor the minor additional electron-donating effect of the alpha-methyl group provide an obvious barrier to oxidation by the heme group of MAO: MDMA has a substantial binding affinity for MAO (undermining steric concerns) and MAO is well known for attacking even tertiary amines (notably N,N-dimethyltryptamine.))
Defensive Strategies
What can an MDMA user do to protect themselves? The first defense is to keep doses within the realm of sanity (nobody should be taking ten pills a night, period, and try to give yourself at least several days between uses.) (See the User’s Guide for more information on dosages.)
Second, be aware of the dangers of overheating. Don’t mix MDMA with drugs that could increase the risk of overheating, such as amphetamines. Don’t mix MDMA with large amounts of drugs that impair mental sharpness (such as alcohol) since, to put it bluntly, being extraordinarily dumb isn’t a pro-survival trait if something does go wrong. If you are going to be dancing (or some other very physical activity) stick to places that are reasonably cool and have good air flow. If you’re going to be dancing or otherwise physically very active for a prolonged period, pay some attention to staying hydrated. (Visit Heatstroke for more information; too much water can be dangerous as well.)
And finally, take some antioxidants. It’s cheap, it’s easy, and may provide a little extra safety margin. (Antioxidant use is actually good advice for any drug user, including smokers and drinkers.) Visit Preloading for more information on antioxidants.
The Prozac Misunderstanding
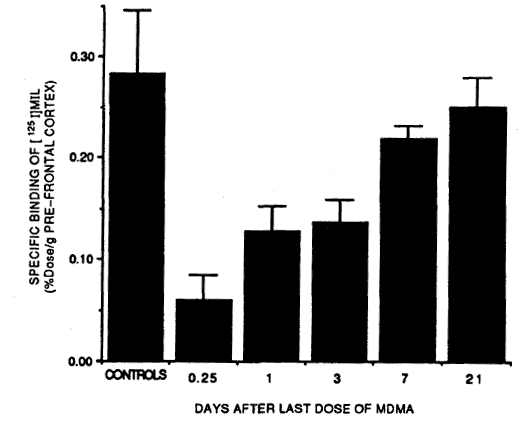
There are also some ‘exotic’ neuroprotective regimens using prescription drugs. SSRIs such as Prozac or Paxil can protect against neurotoxicity by preventing access to the inside of the axon. However, these drugs also partially block MDMA’s effects. There has been a popular belief that taking an SSRI like Prozac up to six hours after taking MDMA can prevent any neurotoxicity from occurring. This is untrue. Here’s a graph from the research that inspired this idea [32]:
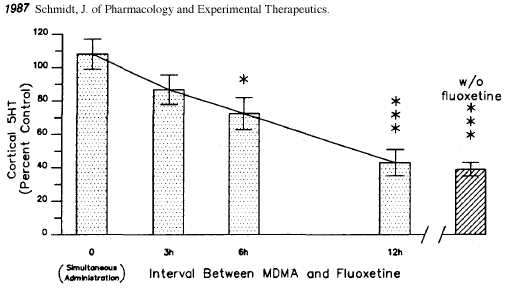
The height of the bars indicates how much serotonin each test group’s brains had one week after the experiment. Lower levels of serotonin indicate neurotoxicity has occurred (since serotonin is made by and stored in the serotonin axons.)
Although giving the rats Prozac (fluoxetine) up to six hours after the MDMA prevented some of the damage, it was only a partial solution. Even at three hours, significant damage appears to have occurred. If there were reason to believe that somebody was beginning to suffer neurotoxic damage (high dose, heatstroke) an SSRI might be an effective ‘rescue’ medication to limit the extent of the damage. However, because ‘post-loading’ with an SSRI is only partially effective, I am of the opinion that the first line of defense against neurotoxicity should be to prevent the ‘neurotoxic scenario’ from happening in the first place through moderation and preventing overheating. Antioxidants are more practical then SSRIs since they can be taken in advance (providing some protection from the start) without interfering with the desired MDMA high (as an SSRI would.)
A more radical approach is the use of the MAOI L-Deprenyl (Selegiline), which has proven to be extremely effective in preventing MDMA neurotoxicity in lab animals and does not interfere with MDMA’s desired activity. In theory, Deprenyl is arguably the most potentially effective neuroprotective regimen, but more work needs to be done before any substantive statement on safety can be made. Several users have reported taking Deprenyl before MDMA with little or no effect (one user reported that Deprenyl made the MDMA dose uncomfortably ‘speedy’.) In order to use Deprenyl as a neuroprotectant, a moderate dose (perhaps 5 mg) should be taken before the MDMA. This combination may prove to be quite dangerous! People experimenting with it should be medically knowledgeable and have a support system in place to provide immediate emergency medical attention if a problem arises.
The Big Question: Is MDMA neurotoxic at recreational doses?
Theories about why and how MDMA might be neurotoxic are fine and good, but we haven’t really answered the most important question of all yet: Are ‘ecstasy’ users damaging their brains? The Drug Enforcement Agency is absolutely adamant that they are, as are many politicians. Where there is a great lack of support for that idea, however, is from the people who should know: Biochemists and neurologists and psychiatrists. To date, I have only seen one group of researchers in the hard sciences (biochem, neurochem, etc.) claim that MDMA users were permanently damaging their brains: Our good friend Ricaurte and his shifting little band of coconspirators.
So what does science have to say about it? There are two basic kinds of research in this area: Studies that directly examine the brain, and studies of how well users are functioning. In the first case, brain scans that can detect SERTs are often used. If serotonin axons have been lost, then the number of SERTs will be lower as well, since they are part of the axons. In the latter case, users are given tests to check their memory, etc. and see how they compare to people who haven’t used MDMA.
‘Ecstasy’ and Memory
Once upon a time, a study of ‘ecstasy’ user’s cognitive function (how mentally sharp they were) came out. It found that, compared to non-users, the ‘ecstasy’ users had slightly worse memory in some areas (mainly word recall.)[6]
The government of course hailed this as proof that the demon-drug ecstasy was destroying poor young minds. However, the study was far less clear-cut than it first appeared. The author (Ricaurte) failed to control for disruptions of sleep patterns (long known to affect cognitive function, and likely to be an issue among stimulant users.) His ‘non-users’ were also younger, better educated, and had higher vocabulary scores than his ‘ecstasy user’ group. The ‘ecstasy’ users also took considerably more of all sorts of drugs than the non-ecstasy users did. They used more opiates, they used more amphetamines…and they smoked considerably more pot, long known to cause (non-permanent) memory problems. Indeed, there were numerous possible explanations for the modest differences in memory, including neuroadaptive responses to recent MDMA exposure.
Marijuana in particular has proven to be a major factor is such test scores. In one study from the Australian government, it was found that, although people who used ‘ecstasy’ and pot did have slightly worse memories than people who didn’t use any drugs, the deficits were in the same places and of the same magnitude as those seen in the group of people that used similar amounts of pot but no ‘ecstasy’. The ‘ecstasy’ users didn’t have poor memories because they were ecstasy users: They had slightly poorer memories because they were unrepentant potheads.[7]
(It should be noted that minor memory problems in the days after use are common, due to disruption of the serotonin system and fatigue. Heavy users also often show some memory and concentration problems, similarly to marijuana (and even alcohol) users. This phenomenon appears to be the result of adaptive changes to the brain, not neurotoxicity.)
Myth: MDMA users have holes in their brains.
The short version is that this is complete nonsense. Like most of the foolishness floating around the planet, this myth started on TV; the Oprah Show to be precise.
As part of their “warning to parents about the Great Evil Ecstasy” show they had a set of brain scans, one of them from a mentally ill girl with a history of past ‘ecstasy’ (and other drug) use:
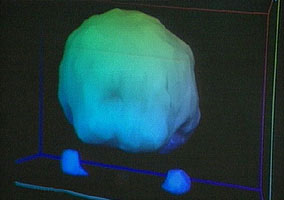
This is the “Healthy Brain” scan shown on Oprah.
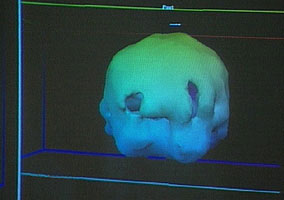
This is the girl’s brain scan.
In spite of appearances, those aren’t holes. This is some form of PET scan; the colors may indicate blood flow or sugar uptake or some more exotic measurement. Where things get tricky is that these renderings don’t show all the data. Instead, the technician will choose which ranges of data are rendered. If they decide to render blood flow from 99%-101% of normal, then an area with 98% of normal blood flow will show up as a gaping hole. The result is that minor differences can be made to look very dramatic, as demonstrated in this rendering of another PET scan:
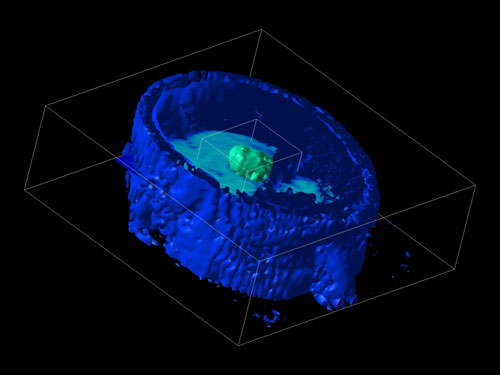
In this case, the technician has ‘removed’ most of the person’s brain by adjusting the computer’s settings. The patient’s face is still largely visible, as is a tumor in the center of their brain. (Perhaps we should start a campaign claiming cancer treatment makes your brain disappear?)
Looking back at the first pair of images, you’ll notice two blobs floating on one side of the ‘healthy’ image. These appear to be the patient’s eyes. The eyes do not appear in the other image, suggesting that different settings have been used–settings that would hide more of the scan’s data. In fact, those two images could possibly have been generated from the same scan of the same person, merely rendered differently.
The girl’s brain scan may actually be abnormal, but there is no way to know what caused it (depression can also reduce brain activity, as can fatigue), or if her brain was ever like the “healthy brain” example they showed, or if what changes were seen were merely temporary.
But, let’s not speculate if science can give us a clear answer to the question. And happily, science does indeed have the answer to whether ‘ecstasy’/MDMA use reduces or alters levels of activity/blood flow within the brain.
The boldest of the brain blood flow experiments was done by researchers from UCLA’s Neurology, Radiology, and Psychiatry departments. They studied the amount of blood flow in current and former ‘ecstasy’ user’s brains and found that recent users (within the past few weeks) had slightly lower blood flow to parts of the brain, but people who hadn’t used for four weeks had perfectly normal levels of total and regional blood flow within their brains.[8] That wasn’t the bold part, however: Not content simply to test volunteers who had used ‘ecstasy’ on their own, they gave recreational doses of MDMA to a number of the volunteers after their initial scan, then re-scanned their brains afterwards. As expected, there was a decrease in blood flow immediately after MDMA use, but it returned to normal within 3-4 weeks. In their final report, the researchers concluded that “Low-dose recreational MDMA use does not cause detectable persistent rCBF (regional cerebral blood flow) changes in humans.”
Further research has only confirmed their findings, so the answer is…yes and no. Yes, there is a modest change to your brain after using MDMA that seems to last for about three to four weeks, give or take. It’s not permanent, and there’s no reason to believe it involves damage; rather, it appears to be a sort of ‘hangover’ effect caused by your brain’s reaction to being exposed to a drug.
Blood flow is significant, because it’s partly dependent on serotonin. If you were really destroying parts of the serotonin system, blood flow should be more-or-less permanently altered. That it wasn’t suggests that these users had not suffered any significant lasting harm from their ‘ecstasy’ use. Additional research on blood flow within the brains of ‘ecstasy’ users has only confirmed the initial findings, even in users who had taken hundreds of pills.[9]
Myth: Human brain scans have proven neurotoxicity.
Again, it’s useful to start with where this all began. In 1998, a piece of research was published that claimed to have found massive neurotoxicity in human ‘ecstasy’ users.[10] The claims they made were so dramatic that the National Institute on Drug Abuse built an ad campaign around it. The most colorful part of the ads (in the literal and figurative sense) was this image graphically showing SERT density:
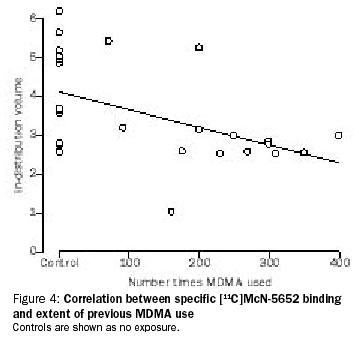
The image isn’t very good, so I’ll explain it a little. The dots are the readings taken from different people. The higher up the dot of a person is, the more SERTs they had.
The group bunched up on the left side of the graph are people who’ve never used ‘ecstasy.’ As the dots get further to the right, the people had used ‘ecstasy’ more times (the record-holder having used on about 400 separate occasions.)
Immediately some questions present themselves. First, the ‘height’ of the graph has been done on a log (ln) scale. What that means is that the data is actually spread out over a lot more space than it appears. Even within the ‘normal’ people (non-users) the top dot is actually over twenty times as far up as the bottom one. Are we to believe that the range of normal for human brains has this much spread in it? Of course not. I may be a foot taller than some people…but I’m not twenty times taller than anybody. The immediate conclusion is that there was something fundamentally flawed in the research. Maybe they did something wrong procedurally. Maybe the brain scan technology they used just didn’t work properly…it was a relatively new technique at the time. Maybe they botched the calculations.
Even if we assume that the brain scans were done properly, and that the data really is trustworthy, there are still some rather large questions. For instance, only one of the ‘ecstasy’ users was actually below the normal range of the non-users, and he was one of the lighter users. How do we know that these people with below average but still normal levels of SERT weren’t simply more likely to take a serotonin releasing drug in the first place? Likewise, two of the volunteers with the highest numbers of SERTs were part of the ‘ecstasy’ user group! If recreational MDMA use destroys serotonin axons (and the SERTs on them), how is it possible that some of the users were well above average?
There is also the small problem of lack of reproducibility. Human brain scan research on ‘ecstasy’ users has continued at an ever-increasing pace since this study, and not one of them has produced anything remotely like these results.
So, we have a data spread that defies belief. ‘Severely damaged’ drug users who were actually normal. And results that nobody else can reproduce. Now…would you care to take a guess as to what miscreant produced this bit of scientifically suspect but loved-by-the-government ‘research’? If you said “George Ricaurte” you’re catching on to the pattern.
The other brain scan work:
Beyond simply not being able to come up with results even remotely similar to what Ricaurte was claiming, the more recent brain scan work is well worth examining in its own right.
Experiment 1: You may recall, near the top of this page, that Ricaurte’s understanding of MDMA has led him to insist that about 1.28 mg/kg would cause neurotoxicity in humans. [2] To say that not many people agreed with him would be an understatement, but again, research is better than speculation.
To lay the groundwork for future human experiments with MDMA in psychotherapy, in 2000 a group of scientists in Switzerland gave a group of human volunteers sensitive brain scans to determine how many SERTs they had. They then gave them 1.5 mg/kg of MDMA (a moderate recreational dose.) A month after they had taken the MDMA, new brain scans were taken. The results? Absolutely no change. They showed no signs whatsoever of neurotoxicity. The researchers concluded that it was “extremely unlikely that a moderate dose of MDMA leads to neurotoxic damage…” [11]
But…1.5 mg/kg isn’t all that much higher than the 1.28 mg/kg Ricaurte swore would cause detectable damage. What about brain scans done on real world heavy users, who frequently push nightly doses to 3 mg/kg and higher?
Experiment 2: The first of the major studies in this field is from a team of scientists in the UK. They studied 10 current ‘ecstasy’ users with an impressive average lifetime usage of about 650 ‘ecstasy’ tablets (one of the volunteers had taken nearly two thousand pills.) The ‘ecstasy’ users were also all amphetamine users; a practice which should increase the risk of neurotoxicity. Much as Ricaurte had, the scientists scanned their brains to see how many SERTs they had, and compared the results to the brain scans of people who had never used ‘ecstasy’, but were otherwise similar in age, sex, education, and other drug use. The results: The ‘ecstasy’ group had slightly lower SERT density. But, the difference was typically on the order of about 2-5%, not the 50-70% losses seen in many of Ricaurte’s rats and monkeys after a single experiment.[12] And, when they examined the data based on the time since each user’s last dose of ‘ecstasy’, something truly fascinating emerged:
If you don’t like reading charts, I’ll explain: The reductions in SERT density depended on how long it had been since the person’s last use of ‘ecstasy’. What changes did occur from ‘ecstasy’ use fully reversed themselves in less than four weeks. That suggests there wasn’t any actual loss of axons…just a temporary reduction in the SERTs on them.
(The data shown was collected from part of the cingulate cortex.)
Experiments 3, 4: Further research has only confirmed what the Brits found. The next pair of experiments come from the Netherlands, conducted by the team of Reneman et al. In their first study, they found that recent ‘ecstasy’ users had slightly lower SERT density when compared to non-users, but former users had normal SERT density.[13] In the second study from this group, the results were striking, both in what they failed to find…and in how the press reported their findings.
This study is one of the largest, most sophisticated pieces of research of its type to date. They gathered precise brain scans measuring SERT density from no less than 54 current and former ‘ecstasy’ users, which they divided into “current moderate”, “current heavy”, and “former” user groups of men and women. The results? The male users didn’t show even temporary reductions in SERT this time, even among the “heavy” user group (which had an average lifetime use of 530 pills.)[14] The female “current heavy user” group, however, did have lower SERT density…but once more, women who where no longer current users had normal SERT densities.
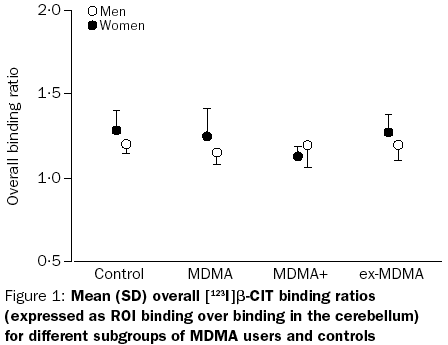

As mentioned above, the moderate users were normal, the heavy male users were normal, the heavy current female users were below average, but former female users were again normal.
So, how do you suppose the press reported this research? Why, they screamed “women at even greater risk from Ecstasy!” Apparently they didn’t think the evidence that the changes were moderate, fully reversible upon quitting, and not even present in the men was a significant piece of information to give the public. But perhaps I judge them too harshly. After all, it’s not as if any of them actually read the research report. New research like this is brought to the attention of the press (and through them, the public) by press releases, which gives the people writing the press releases a great deal of influence over what the public thinks it meant. (This is why I don’t believe in ever using secondary sources like news reports when it comes to research. Only the research itself can be trusted to tell its story accurately, and even then not always.)
However, one might wonder, could this recovery of SERTs mean that neurotoxicity did occur (destroying axons), but the axons regrew? This question has also been answered: When baboons were given a neurotoxic dose of MDMA (destroying many of their serotonin axons), then given brain scans for SERT density a year later, axon regrowth was seen. However, the new axons that had grown didn’t get far from the center of the brain. As a result, parts of the brain near the raphe nuclei (the clusters of serotonin neurons in the base of the brain) had much higher levels of serotonin axons than the brains of normal baboons, but the more distant parts of their brain remained depleted of serotonin axons.[15] Monkey experiments produced similar results.[16] The normal distribution of SERTs in the ‘recovered’ human users cannot be explained by axon regrowth; the research only fits if we assume that the axons were not destroyed. Somehow, MDMA use (at least at heavy levels) can cause temporary reductions in the number of SERTs on the axon, but the axons themselves appear to have been spared.
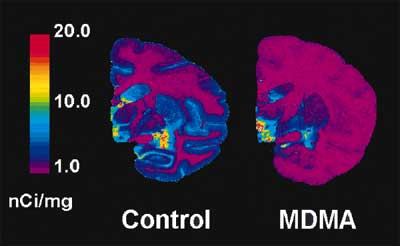
This is your baboon on drugs: SERT density measured by PET scan. The image on the right is representative of the baboons that were given a neurotoxic dose of MDMA a year before the brain scans; the animal on the left was not given any drugs.
How MDMA exposure can cause reduced SERT density isn’t clear. It’s possible that regular exposure, by depleting serotonin, signals the serotonin neurons to produce fewer SERTs (a phenomenon that has been demonstrated with other serotonin-depleting drugs.[33]) It’s also possible that the axons make fewer SERTs available on their surface as a response to over-activation during MDMA exposure. It could also be that the oxidative strain placed on the axon by MDMA’s breakdown (if MDMA itself is indeed the culprit) is enough to increase the rate of SERT breakdown, causing levels of SERT to decline as ‘wear and tear’ on them exceeds the rate of replacement. Regardless of the mechanism, SERTs come back as long as the axon is still there…and there is good reason to believe the axons are still there in these people.
Experiment 5: Beyond the above work finding no lasting harm from MDMA, the original ‘doom and destruction’ Ricaurte experiment has been replicated. In 2003, a group of German researchers working for the German version of the FDA published the results of a massive brain scan study on current and former ecstasy users.[17] The German group, composed of experts in radiology and neurology, used the SERT tracer “(+)McN5652” and PET scans, just as Ricaurte had. Where Ricaurte had 14 ecstasy users with an average use of 880 tablets, the German group had 29 current users with an average of 827 tablets and 29 former users with an average use of 793 tablets.
The results are shown in the graph below. Current ecstasy users had slightly lower SERT density than non-users, but the former ecstasy users were indistinguishable from people who had never used the drug:

So, how it possible that the original piece of Ricaurte research, so vaunted by the US government, trumpeted from every news outlet, and chronically referred to in prohibitionist literature as proof of the evils of MDMA was so badly off? Maybe the gods smiled on him and brought him subjects that simply were not representative of the general population. Maybe he and his people screwed up somehow. And maybe…maybe he simply took a ‘creative’ view towards data collection methods. We may never know for sure. In the meanwhile, when the minions of prohibition scurry about speaking of the ‘evils of ecstasy’, they will no doubt continue to refer to Ricaurte’s work as their ‘proof’, ignoring the newer, larger, more sophisticated, and much more numerous experiments suggesting that changes to the average ecstasy user’s brains from common patterns of use are, at worst, minor and temporary.
Experiment 6: Neuroendocrine Challenge. One of the long-term effects of MDMA neurotoxicity in lab animals is that the animals become hyper-sensitive to drugs that activate serotonin receptors. This occurs because when levels of serotonin severely decrease, the brain increases the number of serotonin receptors to try to compensate. As a result, when the animals are given serotonin-receptor activating drugs, the drugs affect them much more strongly than they would animals who’s serotonin systems were undamaged. By measuring levels of a ‘stress hormone’ called prolactin in the blood we can gauge the intensity of the animal’s reaction to serotonin receptor activating drugs, such as m-CPP (m-chlorophenylpiperazine.) The graph below shows the difference in reaction to m-CPP in normal monkeys vs. ones that had been given a neurotoxic dose of MDMA years earlier:
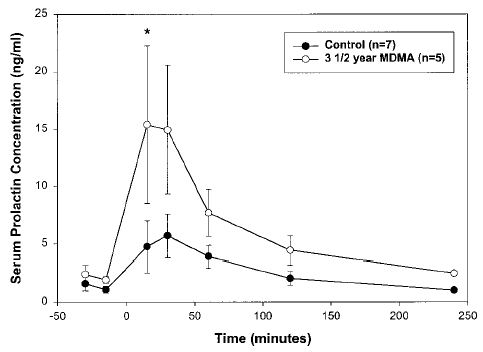
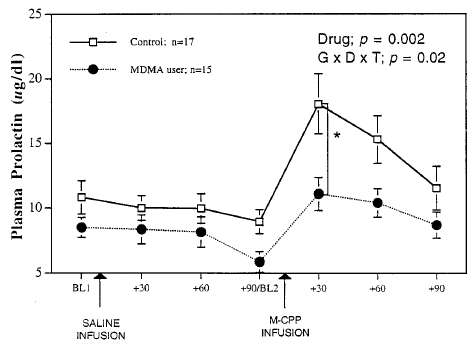
That they were actually somewhat less stressed by the drug seems surprising at first…but becomes less odd when you consider that, by definition, MDMA users are people who use and like drugs that activate serotonin receptors (in the case of MDMA, by releasing serotonin.) The ‘ecstasy’ users also smoked more pot, which other research has found also reduces the prolactin response to serotonin receptor activation.[36]
An argument could be made that the ‘ecstasy’ users brains might have suffered neurotoxicity but were not sensitized because they had downregulated serotonin receptors in response to recent drug exposure. However, this argument falls short on several counts. First, the ‘ecstasy’ users had an average of 14 weeks between their last dose and the test. Rats show complete re-setting of serotonin receptor densities within 3-4 weeks of their last dose of MDMA.[37] Although humans are likely to take more time to recover from a pharmacologically equivalent dose than a rat, 14 weeks is somewhat of a stretch; other research has found that users who had been abstinent for 20 weeks had comparable serotonin receptor densities to non-users.[38] Secondly, receptor downregulation requires that the receptors be overactivated in the first place. If the recreational doses of MDMA used repeatedly in the past by these individuals were neurotoxic, how was it possible that ‘ecstasy’ use had been able to release enough serotonin from non-existing axons to cause downregulation?
Ricaurte’s great Ecstasy Parkinson’s Fraud:
In the fall of 2002 George Ricaurte, the Dark Prince of suspect science, published an article in the prestigious journal Science that claimed that a “common recreational dose” of MDMA caused severe damage to the dopamine systems of monkeys.[26] (Parkinson’s disease involves the death of dopamine neurons, so extensive damage to your dopamine axons could presumably cause symptoms similar to Parkinson’s.) The press and politicians went mad. Draconian new anti-MDMA laws were passed, including the infamous RAVE Act, which made it illegal to throw a party if you “should have known” that some people would be using drugs at it. MAPS, which had been on the verge of finally having its MDMA post-traumatic stress disorder research approved, was stopped cold in the face of the apparent new evidence of MDMA’s horrific dangers.
More than a year later, after scathing criticism from TheDEA.org, the house of cards collapsed when Ricaurte sheepishly admitted that the experiment never really happened: The monkeys had actually been given massive doses of methampetamine, not MDMA! He also admitted that no matter what they had tried, they had been unable to damage monkey’s dopamine systems with real MDMA.
As the saying goes, it’s all over but the shouting. Ricaurte claims the drug manufacturer switched the labels on two vials (one of methamphetamine, one of MDMA) causing the error. The manufacturer (RTI) has vigorously disputed the claim that a switch occurred at their facilities (which are very tightly run under D.E.A. oversight.) The study reportedly cost the American taxpayers $1.3 million. Somebody owes us a refund; I nominate Ricaurte.
• MAPS coverage of the scandal (including links to the original article, the retraction, and reactions from the research community.)
And of course, there is my own not-so-little contribution to the shit-storm:
TheDEA.org's letter to Science, sent in July of 2003. Over the next two months, the journal asked Ricaurte to re-examine his findings, leading to the bombshell 'discovery'.
To the Editors of “Science” Magazine:
Regarding the research report entitled “Severe Dopaminergic Neurotoxicity in Primates After a Common Recreational Dose Regimen of MDMA (“Ecstasy”)”, published in Science in September of 2002:
The authors (Ricaurte et al) casually refer to a case report as an example of “MDMA-induced Parkinsonism.” The patient in question was a young man who had reported infrequent use of the drug (a total of ten exposures) over the course of the past year.[1] He had not used “ecstasy” for approximately three months when he presented for the symptom of “slight clumsiness in upper and lower extremities.” Over several months following initial presentation for treatment, his condition rapidly worsened, developing more features characteristic of Parkinson’s. The patient did not respond to “the maximal tolerated doses of levodopa and pramipexole”, leaving the fundamental assumption of damage to the dopaminergic system as the source of the patient’s problems very much in doubt.
The doctors who reported this case state that “we have no firm evidence of a causal relation between this patient’s drug use and his parkinsonism”; their belief that it may have been caused by infrequent MDMA use seems to be born almost entirely of their inability to make an alternative diagnosis coupled with MDMA’s known dopaminergic neurotoxic potential in mice.
The pathology of this case (delayed onset relative to drug use followed by rapid progression) is inconsistent with all known research on the progression of MDMA neurotoxicity, which produces a maximization of damage shortly after exposure followed by slow partial recovery.[2]
If Ricaurte et al have additional information about this case that was unavailable to the doctors treating the patient, they have made no mention of it. At the least, it was dishonest to characterize this case simply as an example of “MDMA-induced Parkinsonism” with no mention of the extraordinarily tenuous and speculative nature of the proposed causal link between the patient’s drug use and his neurological symptoms.
Equally intriguing is the author’s claim that “oral administration offers little or no significant neuroprotection” relative to the injected route used in the experiment. Indeed, Ricaurte’s own research has demonstrated that injecting MDMA can double and even triple it’s neurotoxicity (depending on brain region) vs. oral dosing.[3] While I will concede that “little or no” is not a clearly defined expression, increases of whole multiples seems to strain any common definition.
Indeed, the very title of the report is rather difficult to defend, as it describes the dose used as “a common recreational dose” of MDMA. If the dose was pharmacologically equivalent to doses routinely taken by humans, why do the authors report that 20% of their animals died on the spot, while an additional 20% may have died had they not been exempted from the full regimen after showing serious distress from only part of the total 6 mg/kg dose? While dosages of such scale do (infrequently) occur in human users, it is likely that these users have reached such dosages in response to growing drug tolerance; with such progressive elevations of dosage over time, neurotoxic potential is reduced.[4] It seems unlikely to me that a man of Ricaurte’s standing within the field of MDMA neurotoxicity was unaware of this, yet no mention of this confound is made in the author’s rush to claim equivalency between their experiment and human patterns of use.
The authors report that 6 mg/kg of MDMA total (2 mg/kg every two hours for 3x) produced profound (approx. 50%) damage to the dopaminergic system in monkeys, but fail to explain why Ricaurte’s own past work has found that a cumulative dose of 40 mg/kg (5 mg/kg at a time 2x for four days) failed to produce any dopaminergic toxicity in monkeys.[5] At the very least, producing a vast increase in toxicity by giving 2 mg/kg 3x instead of a single 5 mg/kg dose seems to demand some discussion; it suggests that at the least the authors have managed to come upon an atypically toxic dosing regimen. If only on the grounds of the sheer novelty of the results from this particular dosage regimen it seems presumptuous to declare equivalency to human users until some explanation can be offered for the sharp divergence of results between these two experiments.
Also of no small concern in the author’s suggestions of severe dopaminergic toxicity commonly occurring in humans is their apparent refusal to mention retrospective studies of the brains of “ecstasy” users that found no differences in their dopamine system relative to non-users.[6][7] While the latter study may not have yet been available to the authors at the time of publication, surely they should have mentioned that there was existing evidence of the absence of harm to user’s dopamine systems before publishing a paper that so shamelessly encourages public panic.
The principle author (Ricaurte) has long championed a minimalistic method of interspecies dosage scaling between non-human primates and humans. His understanding of MDMA neurotoxicity has led him to believe that less than 1.3 mg/kg of MDMA in a human user would produce neurotoxic damage (a dose he arrived at by scaling from 5 mg/kg in non-human primates, which he reports as producing neurotoxicity.)[8] If we believe Ricaurte’s claims of dosage scaling between humans and non-human primates, then the 6 mg/kg dose used in his recent experiment (which killed a significant number of his animals and severely damaged the serotonergic and dopaminergic pathways in the survivors) would be equivalent to just over 1.5 mg/kg in humans. However, prospective human brain-scan experiments have failed to find even the slightest indication of neurotoxicity (or even subject distress) at 1.5 mg/kg.[9] Human users routinely exceed this dosage, mix drugs, and use under uncontrolled and adverse conditions, yet fatalities are rare. Indeed, examinations of the brains of current human “ecstasy” users with an average lifetime exposure of ~800 tablets found only minor reductions in SERT (serotonin transporter) density, and when abstinent users were examined, their regional and total SERT densities were indistinguishable from non-drug users.[10] While regrowth of serotonin axons could explain a recovery of total SERT density, the recovery seen in these heavy human users was uniform, precisely restoring normal SERT density in all regions of the brain studied, as contrasted with the patterns of serotonin axon regrowth seen in non-human primates given neurotoxic doses of MDMA (which produced hyperenervation of regions near the raphe nuclei but failed to restore enervation to less proximal regions.) Given this pattern of recovery, there is every reason to believe that the minor temporary loss of available SERT proteins observed was not due to neurotoxic damage (destruction of axons.)
Ricaurte also fails to explain why his “common [human] recreational dose” of MDMA produced a significant and prolonged (even lethal) hyperthermic response (as much as 41.6C) when human experiments have consistently produced little or no hyperthermic response at any dose attempted.[11][12] As hyperthermic response is well-established as a critical factor in MDMA neurotoxicity[13], I must again question why Ricaurte believes the dosages he uses are pharmacologically equivalent to those used by humans when the physiological responses produced are not equivalent. It’s certainly true that malignant hyperthermia appears with some frequency among human users, but such cases are usually associated with prolonged dancing at clubs. Are we to believe that Ricaurte et al. threw a ‘rave party’ for their animals?
In the final analysis, I must regard this recent product of Ricaurte et al. as more of an act of propaganda than a sincere attempt to advance public understanding. There is no reason to believe that there is a coming wave of ‘Ecstasy Parkinsonism’ among human “ecstasy” users, or even that common patterns of use pose a risk of injury to the dopaminergic system. Shame on the authors for the incomplete, misleading and sensationalistic nature of this research report, and shame on the editors of Science for publishing it.
[1] Mintzer S, Hickenbottom S, Gilman S “Parkinsonism after taking ecstasy” N Engl J Med, 1999; 340(18):1443. Abstract.[2] Scanzello CR, Hatzidimitriou G, Martello AL, Katz JL, Ricaurte GA “Serotonergic recovery after (+/-)3,4-(methylenedioxy) methamphetamine injury: observations in rats” J Pharmacol Exp Ther, 1993; 264(3):1484-91. Abstract.
[3] Ricaurte GA, DeLanney LE, Irwin I, Langston JW “Toxic effects of MDMA on central serotonergic neurons in the primate: importance of route and frequency of drug administration” Brain Res , 1988; 446(1):165-8. Abstract.
[4] Frederick DL, Ali SF, Slikker W Jr, Gillam MP, Allen RR, Paule MG “Behavioral and neurochemical effects of chronic methylenedioxymethamphetamine (MDMA) treatment in rhesus monkeys” Neurotoxicol Teratol, 1995; 17(5):531-43. Abstract.
[5] Ricaurte GA, Forno LS, Wilson MA, DeLanney LE, Irwin I, Molliver ME, Langston JW “(+/-)3,4-Methylenedioxymethamphetamine selectively damages central serotonergic neurons in nonhuman primates” JAMA, 1988; 260(1):51-5. Abstract.
[6] Semple DM, Ebmeier KP, Glabus MF, O’Carroll RE, Johnstone EC “Reduced in vivo binding to the serotonin transporter in the cerebral cortex of MDMA (“ecstasy”) users”, British Journal of Psychiatry, Vol 175, 63-69 (1999). Abstract.
[7] Reneman L, Booij J, Lavalaye J, de Bruin K, Reitsma JB, Gunning B, den Heeten GJ, van Den Brink W “Use of amphetamine by recreational users of ecstasy (MDMA) is associated with reduced striatal dopamine transporter densities: a [123I]beta-CIT SPECT study– preliminary report” Psychopharmacology (Berl), 2002; 159(3):335-340. Abstract.
[8] Ricaurte GA, Yuan J, McCann UD “(+/-)3,4-Methylenedioxymethamphetamine (`Ecstasy’)-Induced Serotonin Neurotoxicity: Studies in Animals” Neuropsychobiology, 2000; 42(1):5-10. Abstract.
[9] Vollenweider FX, Gucker P, Schönbächler R, Kamber E, Vollenweider-Scherpenhuyzen MFI, Schubiger G, Hell D “Effects of MDMA on 5-HT uptake sites using PET and [11C]-McN5652 in humans” Conference of the German Society for Psychiatry, Psychotherapy and Neuromedicine, 2000. Abstract.
[10] Buchert R, Thomasius R, Nebeling B, Petersen K, Obrocki J, Jenicke L, Wilke F, Wartberg L, Zapletalova P, Clausen M “Long-term effects of “Ecstasy” use on serotonin transporters of the brain investigated by PET.” J Nucl Med 44: 375-84 (2003). Abstract.[11] Mas M, Farre M, de la Torre R, Roset PN, Ortuno J, Segura J, Cami J “Cardiovascular and neuroendocrine effects and pharmacokinetics of 3, 4-methylenedioxymethamphetamine in humans” J Pharmacol Exp Ther, 1999; 290(1):136-45. Abstract.[12] de la Torre R, Farre M, Roset PN, Hernandez Lopez C, Mas M, Ortuno J, Menoyo E, Pizarro N, Segura J, Cami J “Pharmacology of MDMA in humans” Ann N Y Acad Sci, 2000; 914:225-37. Abstract.[13] Yuan J, Cord BJ, McCann UD, Callahan BT, Ricaurte GA “Effect of depleting vesicular and cytoplasmic dopamine on MDMA neurotoxicity”, Journal of Neurochemistry, Vol 80, 960-969 (2002). Abstract.
Ecstasy use and mental health.
There’s been a news story claiming that ecstasy use causes depression. The research this story is based on has apparently never been published, suggesting that it was of such low quality that even in an age of anti-drug hysteria no scientific publication felt it was worth printing. However, the basic finding (that ecstasy users are more likely to have emotional problems) does in fact appear to be true.
In a large longitudinal German study (tracking about 2,500 people over time) it was found that, at the end of the study, 68% of those reporting having used “ecstasy” at least once also reported having suffered from a DSM-IV psychological disorder at some point in their lives.[18] (DSM-IV is a mental health diagnostic manual.) However, the researchers report that in about 90% of these cases, the person was diagnosed with a mental disorder before their first use of ‘ecstasy.’ It is likely that MDMA (and other drug use) can trigger or worsen psychiatric problems, but in at least the vast majority of cases, psychiatric problems are more likely to be the cause of the drug use, not the other way around. ‘Ecstasy’ users, statistically speaking, are not normal to start with.
• Visit the Mental Health page for more information.
“But I know people that are stupid/emotionally unstable from using ‘ecstasy’…doesn’t that mean they’ve been brain damaged?!”
Not necessarily. The nervous system tries to correct for unusual levels of activity, often by ‘turning the volume down’ (or up) on different systems. In the case of coffee drinkers, your brain makes itself less sensitive to caffeine, creating tolerance (and in many cases, mild dependence.) If you take a lot of acetaminophen, your body eventually works to counteract it, which can lead to ‘rebound’ headaches (the headache coming back if you stop taking acetaminophen.) This phenomenon of your brain trying to compensate for unusual factors (such as drugs) is called neuroadaptation, and is one cause of drug tolerance (needing more to have the same effect) and physical dependence. Neuroadaptation is not damage! It is a constant, normal, and entirely controlled process by which your brain tries to keep itself running smoothly.
Sometimes neuroadaptation can be manipulated in useful ways: SSRI antidepressants like Prozac, Zoloft, Paxil, etc. work partly by getting your brain to change its sensitivity to serotonin (for those drugs the process is somewhat slow, which is why it takes several weeks for an SSRI to become fully effective against depression.)
However, in the case of recreational drug use, this effect is almost always disruptive: You take a drug for a brief period, your brain tries to compensate, and then when the drug is gone your brain is off-balance again, now over-compensating. It will return to normal, but the process can take days, weeks, in some cases even months. In the case of MDMA, your brain makes itself less sensitive to serotonin to try to compensate for all the serotonin the drug releases. Fully re-setting its sensitivity levels after even a single use of MDMA can take weeks, even months from a very high dose. If you use MDMA frequently, your brain doesn’t have enough time to re-set and gets pushed further and further from normal levels of serotonin sensitivity. In extreme cases, this can apparently lead to users becoming seriously depressed, anxious, unmotivated, poor memories, etc.
It’s quite understandable that people are afraid of brain damage when they experience this sort of mental disruption; the average person has never been told that there were any other possible explanations, in spite of virtually all of us being familiar with the basic phenomenon in the form of ‘needing that first cup of coffee in the morning to get going’ and the like. My position is not that people don’t get seriously screwed up by frequent use of MDMA; only that the cause is unlikely to be actual permanent damage. Given a break from use of a few months, even the most severely ‘e-tarded’ user should find themselves greatly improved as the brain slowly returns to its normal ‘volume settings.’ (For more information and an animation of one process of neuroadaptation, visit MDMA At Work.)
Conclusions
I absolutely believe that MDMA can be neurotoxic in humans. There’s just no reason to believe that it is at a sane dosage/under normal circumstances, and your odds can be greatly improved with a little common sense: Don’t mix drugs, be aware of overheating dangers, and take some antioxidants.
What I would consider safe usage of MDMA?
• A single dose. Taking multiple full doses in an evening may not be safe. As a single dose, however, even doses high enough to just about knock you on your ass (in the 2 mg/kg range) are probably safe. (‘Safe’ in the sense that driving a car is safe; relative, not absolute safety.)
• A safe environment. That means either moderate (‘room temp’) air temperatures or avoiding high levels of activity (dancing.)
• No drug mixing, including alcohol. Not all drugs can increase risk, but unless you’re sure you know what you’re doing, it’s best not to be on other drugs while high on MDMA.
MDMA-related injuries and deaths are in most cases actually overheating injuries and deaths. MDMA does not normally cause significant increases in body temperature in humans; a significant increase in body temp is abnormal and should be treated immediately.
The role of overheating in MDMA neurotoxicity can hardly be exaggerated; no animal experiment has ever produced neurotoxicity at any dose of MDMA at normal human body temperature. In the infamous Ricaurte “Ecstasy Parkinsonism” monkey experiment, his animals reached body temperatures of as high as 107F. More typically, experimental animals that develop MDMA neurotoxicity reach body temperatures of about 39C (103F). Although the exact mechanisms of MDMA neurotoxicity are at best imperfectly understood, damage is clearly a result of the combination of the unusual strain placed on the neurons by drug exposure being greatly amplified by overheating. I do not anticipate human neurotoxicity at any likely voluntarily taken dose of MDMA in the absence of significant and prolonged hyperthermic response.
Origin of the Species
The research leading to this final document involved a comprehensive review of English-language MDMA research from the 1950s to present; well over a thousand journal articles detailing numerous animal and human experiments, field reports from doctors, opinions of experts, etc. ‘Library research’ of this sort is a rather thankless and tedious task, but when you get to the end, you’ve really gotten to the end; no opinion is left unconsidered, no research left unexamined. I compiled my notes, discussed and argued with other academics, contacted researchers for more information in some cases, etc. This document expresses and makes the case for the final opinion that my research brought me to: That moderate recreational use of MDMA does not present a credible risk for neurotoxicity. It is not a comprehensive discussion of the totality of the research (although most brain scan work, pro and con, has been included.)
Conspicuously lacking is detailed coverage of the numerous generic sorts of ‘ecstasy users not as mentally sharp’ type of research. This was not done as a way to avoid evidence against my position; rather it is because the quality of such research has been so consistently poor as to not merit detailed examination. The typical work in this category makes the following chain of assumptions:
1. People who use ‘ecstasy’ were (prior to their drug use) just like non-drug users.
2. The people we recruit are representative of the larger user population.
3. No other drugs that the volunteers use will affect results.
4. There are no differences in sleep patterns, sub-clinical psychiatric disturbances or any other environmental factors that could affect test performance.
5. Any differences that are found must be due to permanent damage. Neuroadaptation (such as the brain scan research amply demonstrates the existence of) will not be considered.
Assumption 1 has been disproven; pre drug-use rates of mental illness in ecstasy users are higher than those of non-drug users as well as users of other categories of drugs. Assumption 2 cannot easily be disproven, but is unlikely to be true. Assumption 3 is disproven by the unremarkable fact that when concurrent use of marijuana is controlled for, differences in cognitive performance (such as word recall) mostly or entirely vanish. 4. Ravers (attendees of all-night dance parties) are usually the recruitment pool for volunteers, which brings a rather unlikely assumption of otherwise equivalent lifestyles between ecstasy users and non-users. 5. That MDMA causes transient disruptions of memory is well established anecdotally, and should be inferred from research identifying neuroadaptive changes in available serotonin receptor densities in both experimental animals and humans following recent MDMA exposure. Researchers in the ‘ecstasy users have worse memories’ field tend to ignore this problem. Those that do make some effort to control this effect usually do no more than ask volunteers not to use for two weeks and give urine test for drugs the day of testing. However, all research indicates that MDMA can subtly affect the mind for at least 3-4 weeks, and urine drug tests only detect use within the past few days.
A less obvious problem in such human research is subject bias. When testing a new medication, you can’t let the people who you’re testing it on know if they’re getting the real drug or a sugar pill, because expectations can produce a powerful placebo effect; people who expect to feel better inevitably do feel better. Any sort of expectation of what the results will be by the test subjects will throw off those same results. This sort of problem is especially great when testing drug users for cognitive effects, because not only do they know if they’re in the control group or not, they probably have some idea of what the researchers expect the result to be. (For instance, just about anybody you recruit for an MDMA study knows that they are expected to have memory problems.) How can you control for this sort of bias? The honest answer is you can’t, although you can at least try not to promote a bias. The most flagrant act of fraud I’ve seen in this category was a researcher who, before testing some ‘ecstasy’ users to see if they were different from non-users, told them that their MDMA use caused untreatable brain damage that would impair their performance. It takes balls of steel to deliberately shape experimental results like that, but not everybody working in this field has a high sense of scientific integrity.
In the end, I hold a dim view of the genre. Besides, why try to guess at serotonergic damage from behavior when we have perfectly capable brain-scanning technology to directly examine the serotonin system? For these reasons of both quality and directness of the measurement technique this page almost exclusively examines brain scan work. At the end of the day, I am wholly unmoved when Billy Bob the psychologist publishes an article claiming evidence of neurotoxicity because his ecstasy-using volunteers were not identical to his non-drug using volunteers. There is an ocean of confounds that must be bridged to make such conclusions credible, especially in light of the strong evidence of no permanent structural changes in user’s brains. The quality of retrospective human research has, however, been increasing over the years; with luck, the future may bring more substantive work.
[1] Ricaurte GA, DeLanney LE, Irwin I, Langston JW “Toxic effects of MDMA on central serotonergic neurons in the primate: importance of route and frequency of drug administration” Brain Res , 1988; 446(1):165-8. Abstract.[2] Ricaurte GA, Yuan J, McCann UD “MDMA (‘Ecstasy’)-Induced Serotonin Neurotoxicity: Studies in Animals”, Neuropsychobiology, 2000; 42(1):5-10. Abstract.[3] Yuan J, Cord BJ, McCann UD, Callahan BT, Ricaurte GA “Effect of depleting vesicular and cytoplasmic dopamine on MDMA neurotoxicity”, J Neurochem, 2002; 80(6):960-9. Abstract.
[4] Sprague JE, Nichols DE “Inhibition of MAO-B protects against MDMA-induced neurotoxicity in the striatum”, Psychopharmacology (Berl), 1995; 118(3):357-359. Abstract.
[5] Shankaran M, Yamamoto BK, Gudelsky GA “Ascorbic Acid Prevents 3,4-MDMA Induced Hydroxyl Radical Formation and the Behavioral and Neurochemical Consequences of the Depletion of Brain 5-HT”, Synapse 2001; 40:55-64. Abstract
[6] Bolla KI, McCann UD, Ricaurte GA “Memory impairment in abstinent MDMA “Ecstasy” users”, Neurology, 1998; 51(6):1532-7. Abstract.
[7] Simon NG, Mattick RP “The impact of regular ecstasy use on memory function”, Addiction, 2002; 97(12):1523-9. Abstract.
[8] Chang L, Grob C, Ernst T, Itti L, Mishkin F, Jose-Melchor R, Poland RE “Effect of ecstasy (MDMA) on cerebral blood flow: a co-registered SPECT and MRI study”, Psychiatry Research (Neuroimaging Section), 2000; 98:15-28. Abstract.
[9] Gamma A, Buck A, Berthold T, Vollenweider FX “No Difference in Brain Activation During Cognitive Performance Between Ecstasy (3,4-MDMA) Users and Control Subjects: A [H2(15)O]-PET study”, J Clin Psychopharmacol, 2001; 21(1):66-71. Abstract.
[10] McCann UD, Szabo Z, Scheffel U, Dannals RF, Ricaurte GA “Positron emission tomographic evidence of toxic effect of MDMA (“Ecstasy”) on brain serotonin neurons in human beings”, Lancet 1998; 352(9138):1433-7. Abstract.
[11] Vollenweider FX, Gucker P, Schönbächler R, Kamber E, Vollenweider-Scherpenhuyzen MFI, Schubiger G, Hell D “Effects of MDMA on 5-HT uptake sites using PET and [11C](+)McN-5652 in humans” Conference of the German Society for Psychiatry, Psychotherapy and Neuromedicine (2000). Abstract
[12] Semple DM, Ebmeier KP, Glabus MF, O’Carroll RE, Johnstone EC “Reduced in vivo binding to the serotonin transporter in the cerebral cortex of MDMA (“ecstasy”) users” Br J Psychiatry, 1999; 175:63-9. Abstract
[13] Reneman L, Lavalaye J, Schmand B, de Wolff FA, van den Brink W, den Heeten GJ, Booij J “Cortical serotonin transporter density and verbal memory in individuals who stopped taking MDMA: Preliminary findings”, Archives of General Psychiatry, 2001; 58(10):901-906. Abstract.
[14] Reneman L, Booij J, de Bruin K, Reitsma JB, de Wolff FA, Gunning WB, den Heeten GJ, van den Brink W “Effects of dose, sex, and long-term abstention from use on toxic effects of MDMA (ecstasy) on brain serotonin neurons” Lancet 2001; 358(9296):1864-1869. Abstract.
[15] Scheffel U, Szabo Z, Mathews WB, Finley PA, Dannals RF, Ravert HT, Szabo K, Yuan J, Ricaurte GA “In Vivo Detection of Short- and Long-Term MDMA Neurotoxicity–A Positron Emission Tomography Study in the Living Baboon Brain”, Synapse 1998; 29(2):183-92. Abstract.
[16] Hatzidimitriou G, McCann UD, Ricaurte GA “Altered serotonin innervation patterns in the forebrain of monkeys treated with MDMA seven years previously: Factors influencing abnormal recovery” J Neurosci 1999; 19(12):5096-107. Abstract.
[17] Buchert R, Thomasius R, Nebeling B, Petersen K, Obrocki J, Jenicke L, Wilke F, Wartberg L, Zapletalova P, Clausen M “Long-Term Effects of “Ecstasy” Use on Serotonin Transporters of the Brain Investigated by PET”, J Nucl Med 2003; 44: 375-84. Abstract.
[18] Lieb R, Schuetz CG, Pfister H, von Sydow K, Wittchen H “Mental disorders in ecstasy users: a prospective-longitudinal investigation.” Drug Alcohol Depend 2002; 68: 195-207. Abstract.
[19] Schmidt CJ “Acute and long-term neurochemical effects of methylenedioxymethamphetamine in the rat” NIDA Res Monogr, 1989; 94:179-95. Abstract.
[20] Bai F, Lau SS, Monks TJ “Glutathione and N-acetylcysteine conjugates of alpha-methyldopamine produce serotonergic neurotoxicity: possible role in methylenedioxyamphetamine-mediated neurotoxicity.” Chem Res Toxicol, 1999; 12(12):1150-7. Abstract.
[21] Colado MI, Green AR “The spin trap reagent alpha-phenyl-N-tert-butyl nitrone prevents ‘ecstasy’-induced neurodegeneration of 5-hydroxytryptamine neurones” Eur J Pharmacol, 1995; 280(3):343-6. Abstract.
[22] Bleh, ref has gone missing…try again in a month or two if you’re comitted. 🙂
[23] O’Shea E, Easton N, Fry JR, Green AR, Marsden CA “Protection against 3,4-methylenedioxymethamphetamine-induced neurodegeneration produced by glutathione depletion in rats is mediated by attenuation of hyperthermia” Journal of Neurochemistry, 2002; 81(4):686-695. Abstract.
[24] Meyer A, Mayerhofer A, Kovar K, Schmidt W “Enantioselective metabolism of the designer drugs 3,4-methylenedioxymethamphetamine (‘ecstasy’) and 3,4-methylenedioxyethylamphetamine (‘eve’) isomers in rat brain and blood” Neuroscience Letters, 2002; 330(2):193-197. Abstract.
[25] Lim HK, Foltz RL “In vivo and in vitro metabolism of 3,4-(methylenedioxy)methamphetamine in the rat: identification of metabolites using an ion trap detector” Chem Res Toxicol, 1988; 1(6):370-8. Abstract.
[26] Ricaurte GA, Yuan J, Hatzidimitriou G, Cord BJ, McCann UD “Severe Dopaminergic Neurotoxicity in Primates After a Common Recreational Dose Regimen of MDMA (“Ecstasy”)” Science, 2002; 297:2260-2263. Abstract.
[27] Reneman L, Booij J, Lavalaye J, de Bruin K, Reitsma JB, Gunning B, den Heeten GJ, van Den Brink W “Use of amphetamine by recreational users of ecstasy (MDMA) is associated with reduced striatal dopamine transporter densities: a [123I]beta-CIT SPECT study– preliminary report” Psychopharmacology (Berl) , 2002; 159(3):335-340. Abstract.
[28] Mintzer S, Hickenbottom S, Gilman S “Parkinsonism after taking ecstasy” N Engl J Med, 1999; 340(18):1443. Abstract.
[29] 1999 Correspondence from The New England Journal of Medicine, Vol 341, No. 18. Link.
[30] de la Torre R, Farre M, Roset PN, Hernandez Lopez C, Mas M, Ortuno J, Menoyo E, Pizarro N, Segura J, Cami J “Pharmacology of MDMA in humans”, Annals of New York Academy of Sciences 2000; 914:225-37. Abstract.
[31] Liechti ME, Vollenweider FX “Acute psychological and physiological effects of MDMA (“Ecstasy”) after haloperidol pretreatment in healthy humans” Eur Neuropsychopharmacol 2000; 10(4):289-95. Abstract.
[32] Schmidt CJ “Neurotoxicity of the psychedelic amphetamine, methylenedioxymethamphetamine” J Pharmacol Exp Ther, 1987; 240(1):1-7. Abstract
[33] Rattray, Baldessari, Gobbi, Mennini, Samanin, and Bendotti “p-Chlorophenylalanine Changes Serotonin Transporter mRNA Levels and Expression of the Gene Product” J of Neurochem, 1996; 67(2); 463-472. Download as PDF.
[34] Hatzidimitriou G, Tsai EH McCann U D, Ricaurte GA “Altered prolactin response to M-chlorophenylpiperazine in monkeys previously treated with 3,4-methylenedioxymethamphetamine (MDMA) or fenfluramine” Synapse, 2002; 44(1):51-57. Abstract.
[35] McCann UD, Eligulashvili V, Mertl M, Murphy DL, Ricaurte GA “Altered neuroendocrine and behavioral responses to m-chlorophenylpiperazine in 3,4-methylenedioxymethamphetamine (MDMA) users” Psychopharmacology (Berl), 1999; 147(1):56-65. Abstract.
[36] Gouzoulis-Mayfrank E, Becker S, Pelz S, Tuchtenhagen F, Daumann J “Neuroendocrine abnormalities in recreational ecstasy (MDMA) users: is it ecstasy or cannabis?” Biol Psychiatry, 2002; 51(9):766-769. Abstract.
[37] Scheffel U, Lever JR, Stathis M, Ricaurte GA “Repeated administration of MDMA causes transient down-regulation of serotonin 5-HT2 receptors” Neuropharmacology, 1992; 31(9):881-93. Abstract.
[38] Reneman L,, Endert E, de Bruin K, Lavalaye J, Feenstra MG, de Wolff F, Booij J “The acute and chronic effects of MDMA (“Ecstasy”) on cortical 5-HT 2A receptors in rat and human brain” Neuropsychopharmacology, 2002; 26:387–396. Abstract.
